We just run out of gold! What now?!? - part 2


What if gold has run out on Earth? No more gold. Es gibt kein gold. No existe el oro. Zołota niet. Złota nie ma - wyszło. In our search for yellow metal, we’re going to travel in time, reach where mankind barely reached so far, discover fascinating world of microorganisms and try to make modern philosophical stone. We’ll step down below Earth’s top layer, touch the ocean bottom and look in the stars. So join us in an adventure in search for not-so obvious and hidden gold.
In the previous part we attempted to imagine world in which gold has run out. Outlined vision led us to conclusion, we better find some and be quick about it. And so, we have started our journey by learning on great past cosmic events and catastrophes, which allowed us to understand some processes occurring in incredible magnitude. In this part, we are going to take opposite approach by checking upon wonderful world of micro - atoms and bacteria.
Gold is wherever, you look for it – especially in the water
Have to start with outlining some numbers – there are 1 mln grams in metric ton. In geology such would be marked in slightly different units – ppm – parts per million. So 10 ppm would simply equal 10 g/t. Hence 1 g/t equals 1 ppm. Even smaller unit are parts per billion (ppb). And some more statistics to consider - more than 70% of the Earth's surface is covered by seawater, which represents 97% of the global water resources. Remaining 2% is in snow and glaciers. Freshwater composes less than 1% in scale.
Knowing that, we need to realise, there are minor concentrations of gold naturally occurring in substances surrounding us. In seawater there is approximately 0.012 parts per billion (ppb) of gold. In 1990 MIT scientists published study assessing, that there is 1 gram for every 100 mln t. of seawater. Based on this source and using another micro measurement unit which we won’t even dare to explain (they simply are extremely small) - it seems Atlantic and North-East Pacific are just slightly more saturated than Pacific, with both at 50 fmol/l. Mediterranean Sea is at 100-150 fmol/l, due to riverbed dust flux and relatively small water circulation via Gibraltar Strait. US National Ocean Services claim that gold in all seawater would be up to 20 mln t. of gold. However in their calculations they also include plant and animal inhabitants of rich and not-so-well-discovered seaworld, so in reality gold volume will be much less. For gold dispersed in seawater (and inhabiting creatures), these values are so small, that currently any attempt to extract gold from water for industrial and investment purposes is beyond any sensible point of financial viability. In comparison, fresh water contains slightly higher rates at 0.02 ppb.
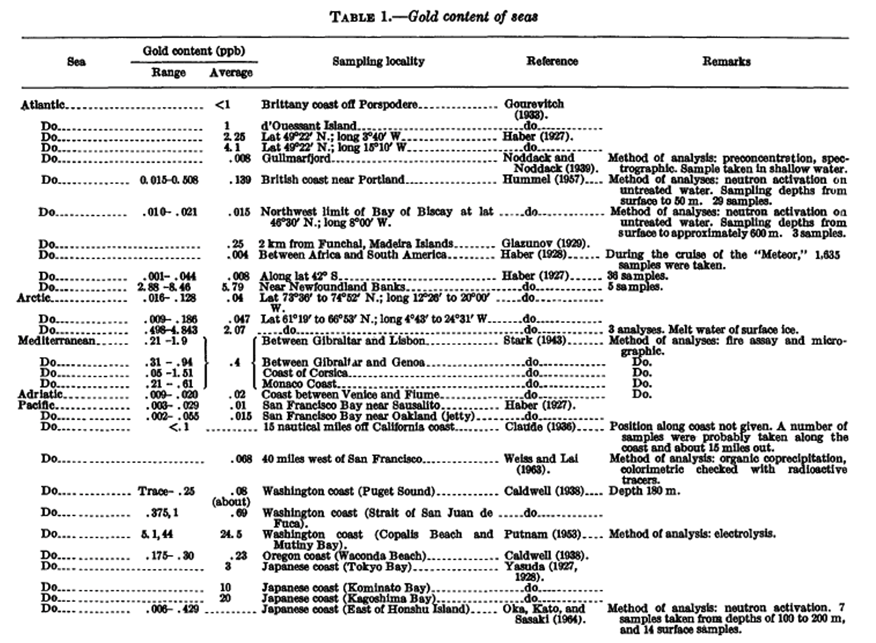
Table presenting seawater sampling for gold content. Source: https://pubs.usgs.gov/circ/1970/0625/report.pdf
In other words, as stated by Edmund Rutkowski – legend of polish gold geology:
‘Gold is wherever, you look for it’
It may just not be in satisfactory amounts…
Gold concentrations in mineable land deposits range from less than 1 to 100 ppm, or higher in bonanza deposits. At 1 ppm concentration of 31 tons of rock must be processed to extract a single ounce of precious gold. Average crustal abundance of gold is at 1.3 ppb, or 0.0013 ppm. But gold is not being evenly distributed and can only be mined profitably where it’s highly concentrated by natural geological, chemical and physical processes. Hence gold in seawater, as highly dispersed at low rate doesn’t seem to qualify for financially extraction.
Nevertheless we tried…
Gold was first detected in seawater in 1872 by the British chemist S. Sonstadt, and ever since, a number of people have promoted the idea of extracting the precious metal from the oceans.sciencedirect.com
In late 19th century a Michigan pastor named Prescott Ford Jernegan tried to attract investors for his gold accumulator invention enabling extraction of yellow metal from water. This eventually appeared to be scam, however crafty pastor and his associate managed to disappear with investor’s money, and had never been seen again.
Another attempt was being made by German chemist Fritz Haber. Nobel Prize winner in Chemistry of 1918 for the invention of Haber-Bosch process. Slightly less famous for his participation in weaponising chlorine gas and other chemical agents for First World War purposes. By extracting gold from water he wanted to help its country in paying war reparations. During development stage, Haber discovered however that his calculations were wrong, and instead of acquiring 65 mg of gold from metric ton of seawater, he could in theory extract 1000% less.
And yet again, another attempts were being made in 1934 by William Dow and in 1941 by Colin Fink – once again turned to be failed. First was owner of Dow Chemicals plant near Wilmington, North Carolina. Plant was constructed to extract the element bromine from seawater for use in gasoline, and was first of its kind. Success in obtaining bromine made Mr. Dow to announce he may be able to extract gold from water. Which turned out to be failure. Former gentlemen – Colin Fink - filed other patent for gold extraction, based on his own bromide extraction process. But once again, there were no reports of success.
A 2002 article in ‘The Hindu’ claimed that Indian scientist Joy Prakash Agarwala had developed a new method for extracting gold as a chemical process. Then biomedical engineer Mark Sullivan had his five minutes, when appeared in tv with an engine that claimed to use the planet’s Coriolis Effect to produce energy. As a byproduct, his oceanic turbines would end up filtering gold from the seawater.
Overall direction of obtaining gold from seawater ended up with massive failures and couple of US registered patents, which in this particular context led to no-achiements.
Good summary of the subject has been published by US Geological Survey report from 1970.pubs.usgs.gov
Seaweed and bacteria seems to be interesting direction for gold recycling
Interesting and more modern approach has been presented with regards to algae. It seems we eventually realised that just energy costs would make separation from seawater financially non-viable. Hence we turned our eyes to nature. Seaweed may be helpful, as in the past we managed to obtain i.e. iodine from seawater seaweed.
Japanese scientists made some tests in early 60’s and reported 0.00015 and 0.00093 ppm gold in the ash of two samples of Ulva sea lettuce. Other samples, one from Tokyo Bay, other from Sagamu Bay, showed 0.000035 ppm and 0.00021 ppm. Unfortunately, this doesn’t differ much from average gold content in seawater. Other interesting experiment had been conducted in 2013 by Pablo Lodeiro and Mika Sillanpaa. Spanish-Finnish team worked on gold recovery from seawater using synthetic materials and seaweed biomass. Results are linked below and they mostly focused on mechanisms of gold recovery. And again there was no breakthrough that could be applied by industry.www.sciencedirect.com
Conclusions is – yes - it is possible to obtain gold nanoparticles. And no –once again we discuss unsatisfactory and financially non adequate volumes. Delftia acidovorans along with Cupriavidus metallidurans are bacteria capable of converting toxic metals into harmless products. Especially first one is being known for its ability to biomineralise gold. First identified in the soil in Netherlands, however in recent years had been also found in other ‘dirty’ environments like sinks and shower drains. These habitats are typically toxic thanks to high concentrations of heavy metals, but Delftia acidovorans thrives in such environment because it contains a peptide known as delftibactin, that neutralizes charged ions. As a result, gold ions are transformed into nanoparticles that eventually accumulate in cracks and crevices as flakes and very small nuggets.www.nature.com
Delftia acidovorans in action. Source: https://www.lib.ncsu.edu/news/dig-into-the-dna-of-delftia%E2%80%94the-microbe-that-poops-gold
So how could we not check possibilities of using our micro friends for obtaining gold? If not from seawater, then maybe of substances and products already containing higher gold content, like electronic scrap?
According to estimates, just in Germany in 2007, about 2 tonnes of gold had been lost, due to inability to recover its particles from industrial sewerage. And this is country, using gold heavily for industrial and chemical purposes, known for its past, now heavily depleted resources. So just try to imagine mining losses occurring in Siberia, Alaska or Nevada.
Idea and above numbers came from a bright Master’s and Bachelor’s students of University of Heidelberg. They formed an interdisciplinary group (biochemistry, molecular biotechnology, pharmacy, and medicine) named iGem Team Heidelberg. In 2013 they conducted tests in controlled environment, on gold plated and gold containing electronic waste. Studies focused on usage of bacteria we already mentioned. While bacteria such as Cupriavidus metallidurans converted dissolved element to its metallic form inside the cell, species like Delftia acidovorans had been used for gold precipitation. Again, lab tests proved to be successful, however apart of scientific publications, there is no further information on any industrial application.( igem.org)
Other lab tests had been conducted by team led by Ahmad Huzaifah Mohd Yusoff from School of Bioprocess Engineering, Universiti Malaysia Perli. Results had been published in 2017. Studies confirmed that Delftia acidovorans is able to process gold chloride (AuCl3), which is the form in which gold exists in seawater. It is toxic for humans, but not for said bacteria. In controlled environment, scientists were able to extract approx. 2.2 g. of gold from pre-prepared gold chloride, and 0.0032 g. from 5 litre of seawater. (iopscience.iop.org)
Conclusion of works were then as follows: Yes, it is possible to extract yellow metal either from gold chloride, seawater or electronic scrap using specialised bacteria. It is officially scientifically proved. However small weights in comparison to resources required and expenditure occurred, make for now such projects financially non-viable and therefore impractical on industrial level. That is of course on assumption Delftia acidovorans won’t be bio-engineered to have its efficiency improved exponentially. There are some promising results with regards to combining its special peptide (delfibactin) responsible for gold recovery skills with E. coli bacteria. However, we’re still far below levels of financial breakeven.
Laboratory for Functional Inorganic Materials (LFIM) claims, that after gold processing and rafination, up to 1% of input material worthy 3.5 bln USD per year is being lost in form of discarded rocks, dust, sludge or waste. Loose translation of this number at 2000 USD, gives us 1.75 mln ounces. 2.6 mln ounces assuming 2018 price of 1300 USD. .
In 2018 its employees, Swiss team of scientists established startup Retreeva in attempt to extract gold and other metals from seawater, but especially from industrial sewerage. Team under Dr. Olga Trukhina and Professor Wendy Queen created – to simplify it – a polymer, metal-organic sponge of unique absorbing structure that works like water filter for type of particles it was designed for. Some successes had been already achieved, in form of 4.7 g. nugget obtained from processing electronics waste.(actu.epfl.chpubs.acs.org)

Retreeva team. Source: https://actu.epfl.ch/news/retreeva-a-startup-that-retrieves-lost-gold-and-cu/
Overall direction seems to be promising, however we’re still discussing laboratory tests and no industrial trials. And only them, could reassure that we experienced major breakout.
Means and ways to split some atoms in most effective way
We know that our whole world is being composed of atoms. Gold is no exception from the rule. Every single atom itself is being composed of core and surrounding electrons. Simplifying we have:
- Neutrons, which are neutral particles located in atom’s core, being part of its mass.
- Protons, which are positive, along with neutron they create atomic nucleus. Number of protons in it equals atomic number - main number based on which elements are being aligned in periodic table. For gold this number is 79.
- Electrons, negative particles circulating around atom’s core. Their mass is very small. So they do not contribute to atomic number.
In atom’s core in theory, we should have equal number of neutrons and protons. In reality, it’s bit different, so bad luck for the theory. Number of protons in element remains constant, however number of neutrons may vary. By adding these two we’ll have mass of our core, also known as isotope. This little difference in isotope caused by different number of neutrons may create significant changes to our element – it may be easily dissolvable in acid, more reactive to other elements or more high temperature resistant. Usually element has one predominant isotope, occurring in nature at 99%+ ratio, and other isotopes composing remaining minority. Gold has just one, single naturally occurring isotope, which is 197. This means, we have 79 protons and 118 neutrons in its core, and it is meaningless if yellow metal was being found in Mongolia, Australia or Peru.
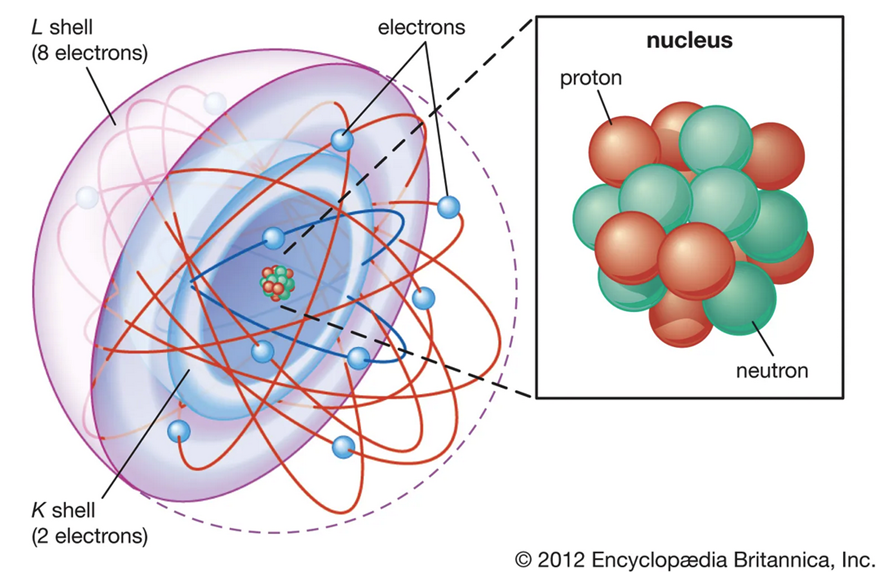
Anatomy of atom. Source: https://www.britannica.com/science/atom
It is possible to synthesize gold and create it with different number of isotopes. Technological advancement of human civilization provided us with tools to do so. And therefore we’re able to synthesize thirty six different gold isotopes, and create yellow metal of atomic mass in between 169 to 205. So is it finally possible to fulfil dreams of past alchemists and create philosophical stone? Or to change lead to gold? Yes and no.
Gold is chemical element with 79 protons in each atomic nucleus. Every atom containing 79 protons is a gold atom, and all gold atoms behave chemically the same. In principle, we can therefore create gold by simply assembling 79 protons (and enough neutrons to make the nucleus stable). Or even easier, we can remove one proton from mercury (which has 80) or add one proton to platinum (which has 78) in order to make gold. Incredibly simply, right? Just on paper.
Adding or removing protons from a nucleus qualifies as nuclear reactions. As such, no series of chemical reactions can ever create gold. Chemical reactions are able to change number and shape of electrons in an atom but they leave nucleus intact. And so, alchemistic dream of creating gold by simply reacting chemicals is therefore impossible. And so, we must focus on nuclear reactions.
The difficulty is that nuclear reactions require a lot of energy. Components of nucleus in a stable atom are very tightly bound together, so it’s hard to get anything permanently into or out of the nucleus. So, to induce a nuclear reaction, we have to shoot high-energy particles at a nucleus.
And now on cons of such solution:
- First of all, gold created this way would be radioactive, and therefore hazardous for human. Decontamination is harder than it sounds because you can't separate out non-radioactive gold from radioactive using purely chemical methods. As such, it couldn’t be sold commercially.
- Second issue would be decay time. Naturally occurring isotope 197 is stable. That means, unless we’ll try to meddle on such atom itself, gold will remain gold. However synthesized yellow metal – in vast majority with different isotope numbers - is unstable and undergoes radioactive decay. In other words, gold turns to metal, just like we’d have reversed philosophical stone. Depending on isotope, this period may take couple hours, days or weeks. Most stable of artificial made gold is isotope 195, which has half-life of 186.1 days.
- Another issue lies in fact, that this process enables to create gold atom. After atom, after atom… Gold is usually measured in troy ounces: 1 troy ounce = 31.1034768 g. And this is when we had to ask physicists for help. Gold has exactly one stable isotope: Au-197 with atomic weight: 196.9666 amu. Avogadro's number tells us on number of particles in 1 mole (or mol) of a substance. For gold that is 6.02214*1023. That is right - twenty-three zeroes. Based on the above we may assume that 1 troy ounce of gold equals 9.51*1022 Au-197 atoms.
- Obtaining troy ounce via nuclear fission, using modern technology would be most energy inefficient absurd to be ever recorded. As stated by Glenn Seaborg – 1951 shared Nobel Prize in chemistry – whose achievement we’ll discuss in next chapter.
To summarise theoretical part on nuclear transmutation of gold, unless we don’t hold two neutron stars in our stashbox and intend to smash them each other, cost to make some gold via nuclear reactions will ascertain heart attack upon reception of energy bill.
Modern alchemists and transmutation wizardry
But yet again we tried – and that is fantastic perk of humankind – curiosity on the world surrounding. About 100 years ago scientists started experimenting with atomic physics, and naturally started to wonder if it’s possible to create yellow metal in this way. After all, at a time money had different values and gold itself was part of monetary system.

Jan Matejko's painting 'Alchemist Sędziwoj'. Depicting the scene of the transformation of a coin into gold by the alchemist Michal Sędziwój. Source: https://pl.wikipedia.org/wiki/Alchemik_S%C4%99dziw%C3%B3j
It all started with first successful nuclear transmutation. In 1901 Frederick Soddy and Ernest Rutherford discovered that radioactive thorium was converting itself into radium. Years later they recalled, they exchanged some thoughts at the moment of realization:
‘Rutherford, this is transmutation!’
-‘For Christ's sake, Soddy, don't call it transmutation. They'll have our heads off as alchemists.’
In terms of successful attempts on creating gold, first seems to be physicist - Hantaro Nagaoka – who managed to lead the way in 1924. Apparently his interest in atomic physics had been ignited, when he heard lecture by Marie Curie-Sklodowska on First international Congress of Physicists in Paris in 1900. In 1924 he managed to synthesize radioactive gold by neutron bombardment of mercury (80 protons). Without prior knowledge of his discovery, team of American scientists - Sherr, Bainbridge, and Anderson - conducted same type of tests in 1941 and managed to created three gold isotopes.journals.aps.org
It is said that, in 1972, Soviet physicists at a nuclear research facility near Baikal Lake opened up the lead shielding of their experimental reactor - and were stunned to discover that its inner surface had turned into gold. Considering secrecy and paranoia of Soviet State, Siberia, scientists, experiment… doesn’t it just sound like urban myth? Pardon our scepticism.
More than 30 years ago, nuclear scientists from Lawrence Berkeley National Laboratory - K. Aleklett, D. J. Morrissey, W. Loveland, P. L. McGaughey, and G. T. Seaborg - succeeded in producing very small amounts of gold from bismuth. Both elements originally have just one stable isotope. Inducted collision caused reaction, they produced a range of gold isotopes from 190 (79 protons and 111 neutrons) to gold 199 (79 protons, 120 neutrons). Researchers reported that in March 1981 on Physical Review C, which we linked below.journals.aps.org
Amount of gold produced was so small they had to identify it by measuring radiation over the course of a year. But - in addition to several radioactive isotopes of gold, particle collisions presumably produced some amount of the stable isotope gold 197. So small volumes, it requires spectrometer to discover.

Glenn Seaborg in 1980. Source: https://achievement.org/achiever/glenn-t-seaborg-ph-d/
So we finally have small amount of stable 197 isotope gold. Problem once again lies in costs. Walter Loveland – one of participants recalls that running particle beams at Lawrence Berkeley National Laboratory required energy usage at add/take 5k USD an hour. And scientists had to use full day of beam time. Glenn Seaborg another participant, who shared 1951 Nobel Prize in Chemistry for his work with heavy elements was senior author on the resulting study. In the interview with Associated Press at a time he concluded on costs:
“It would cost more than one quadrillion dollars per ounce to produce gold by this experiment."
Price per ounce at the time was at 560 USD.
To be continued…